Glycomaterial-based formulation outperforms superabsorbent polymer in menstrual care

What Has Been Achieved:
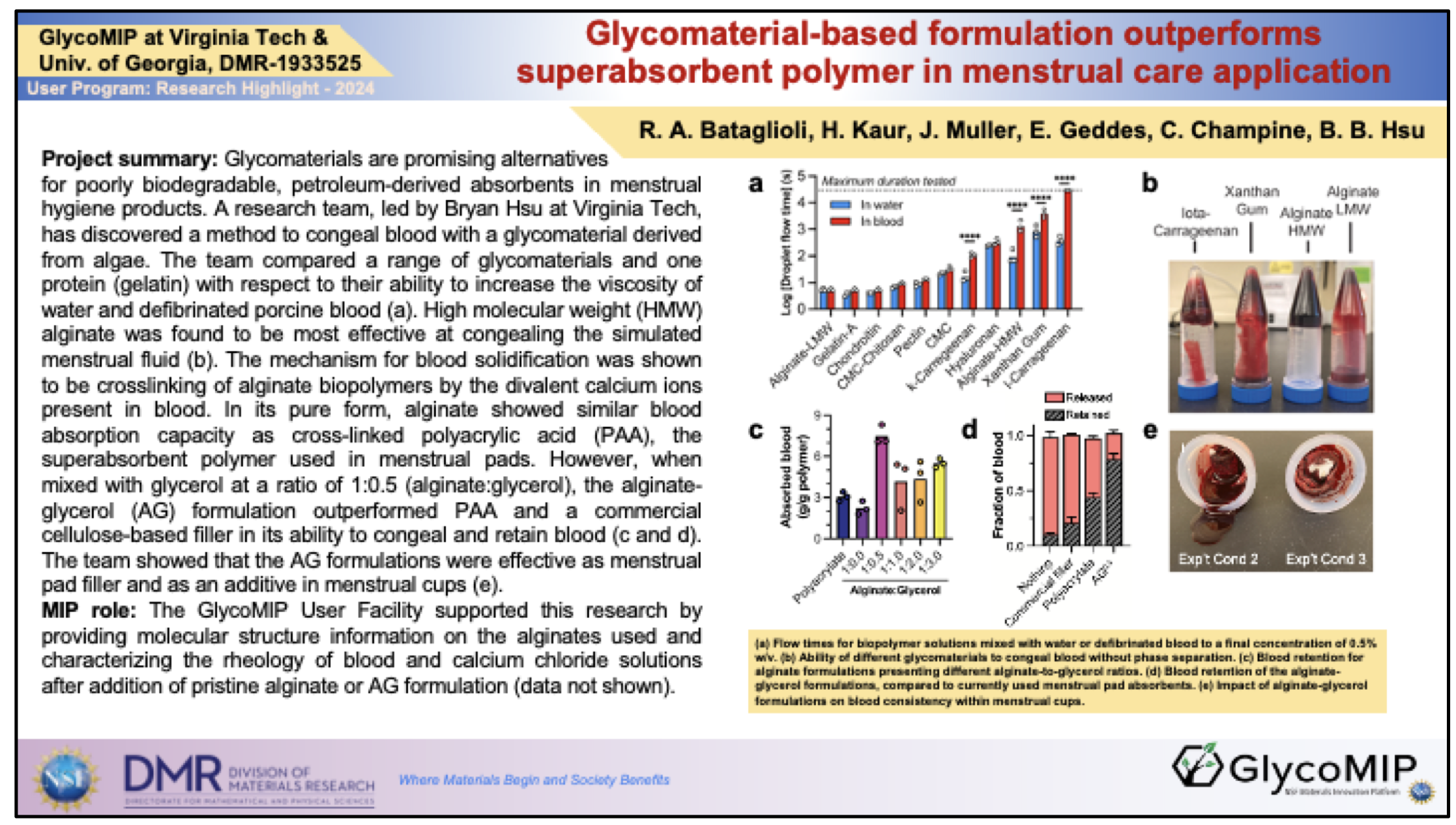
A team of researchers, led by Bryan Hsu at Virginia Tech, developed a novel glycomaterial-based powder formulation, combining alginate and glycerol, that effectively congeals blood. The formulation outperformed currently used materials, specifically a cellulose-based menstrual pad filler and superabsorbent polyacrylic acid, in both menstrual pad and menstrual cup applications. The publication is in the top 5% of all research outputs scored by Altmetric and has been mentioned by 24 news outlets, 21 X users, and 2 Facebook pages.
Importance of the Achievement:
The novel alginate-glycerol formulation, composed of natural, non-toxic, biodegradable components, is a promising alternative for poorly biodegradable, petroleum-derived polyacrylic acid in menstrual hygiene products.
Unique Feature(s) of the MIP that Enabled this Achievement:
The research was enabled by GlycoMIP’s unique expertise and capabilities to characterize both the molecular and material properties of alginates and its solutions and gels.
Publication:
Bataglioli, R. A.; Kaur, H.; Muller, J.; Geddes, E.; Champine, C.; Hsu, B. B. A naturally derived biomaterial formulation for improved menstrual care, Matter 2024, 7(9), 2941-2958. DOI: https://doi.org/10.1016/j.matt.2024.06.028
Local User Project:
230926-RP - Polysaccharide based biomaterial for improved menstrual health and hygiene
Entire acknowledgement statement:
The authors would like to thank Hollyn Franklin, Rita Makhlouf, Sydney Murphy, and Abigail Heimbach for their assistance with in vitro menstrual cup testing; Ryan Porell and Caylyn McNaul at the GlycoMIP facility for rheological testing and polymer char- acterization; and Hiba Baaziz, Krisangel Lopez, and Analis Smith for insightful discus- sions. The contact-angle measurements were made possible by the use of Virginia Tech’s Materials Characterization Facility, which is supported by the Institute for Critical Technology and Applied Science, the Macromolecules Innovation Institute, and the Office of the Vice President for Research and Innovation. Funding was pro- vided to B.B.H. by award no. 208-03-22 from the Commonwealth Health Research Board of Virginia. Funding to the GlycoMIP was provided by the National Science Foundation Materials Innovation Platform through Cooperative Agreement DMR- 1933525. Funding to R.A.B. was provided, in part, by the Postdoctoral Fellowship in Drug Delivery from the PhRMA Foundation. Some figures were created with Biorender.com.